|
October 2022 TARGETING ION CHANNELS TO MANAGE OSTEOARTHRITIS PAINFeaturing: Paul DeCaen, PhD
Therapeutically targeting ion channels in nociceptor neurons may be a promising strategy to manage osteoarthritis pain, according to a Northwestern Medicine study published in Cell Reports.
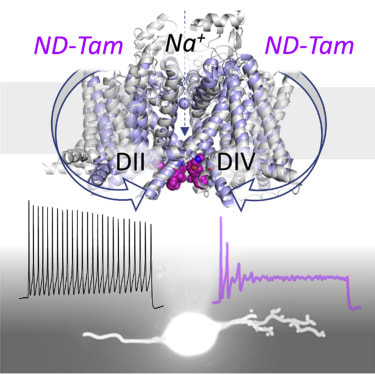
Paul DeCaen, PhD, associate professor of Pharmacology, was senior author of the study. Nociceptor neurons transmit pain signals to the brain using electrical currents generated by voltage-gated sodium channels; when overactive, those channels are commonly observed in cases of osteoarthritis and other aberrant pain conditions. Targeting the sodium channels to improve osteoarthritis pain management is been explored as a potential therapeutic strategy. Previous work from the DeCaen laboratory demonstrated that a metabolite of tamoxifen, an estrogen receptor modulator, binds to the intracellular exit of the channel, underscoring it’s therapeutic potential. Despite the clear need, efforts in developing novel sodium channel blockers into real therapies has been challenging, according to DeCaen. “Repurposing tamoxifen metabolites, commonly used to treat breast cancer, presents an alternative strategy which can bypass phase one clinical trials,” DeCaen said.
In the current study, DeCaen’s team administered a tamoxifen metabolite to mice that had been genetically modified to develop osteoarthritis symptoms. They found the drug bound to two intracellular receptor sites in sodium channels, blocking the entry of ions using a “bind and plug” mechanism and inhibiting sodium channels in inflamed, overactivated nociceptor neurons responsible for osteoarthritic pain. “To our knowledge, clinical efficacy of tamoxifen metabolites for osteoarthritic pain treatment is untested. Yet many years of oncology clinical data significantly de-risks their use, which may lower overall development costs for their use to treat osteoarthritic pain,” DeCaen said. Megan Larmore and Eduardo Guadarrama, students in the Driskill Graduate Program in Life Sciences (DGP), and Thuy Vien, PhD, research assistant professor of Pharmacology, were co-authors of the study. This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases grant 1R01 DK123463-01 and National Institute of General Medical Sciences grant 5T32 GM008382. This article was originally published in the Feinberg School of Medicine News Center on October 4, 2022. |
Paul DeCaen, PhD, associate professor of Pharmacology, was senior author of the study published in Cell Reports.
Refer a PatientNorthwestern Medicine welcomes the opportunity to collaborate with you in caring for your patients.
|
You May Also Like
|
February 2022 |
November 2021 |
September 2021 |