|
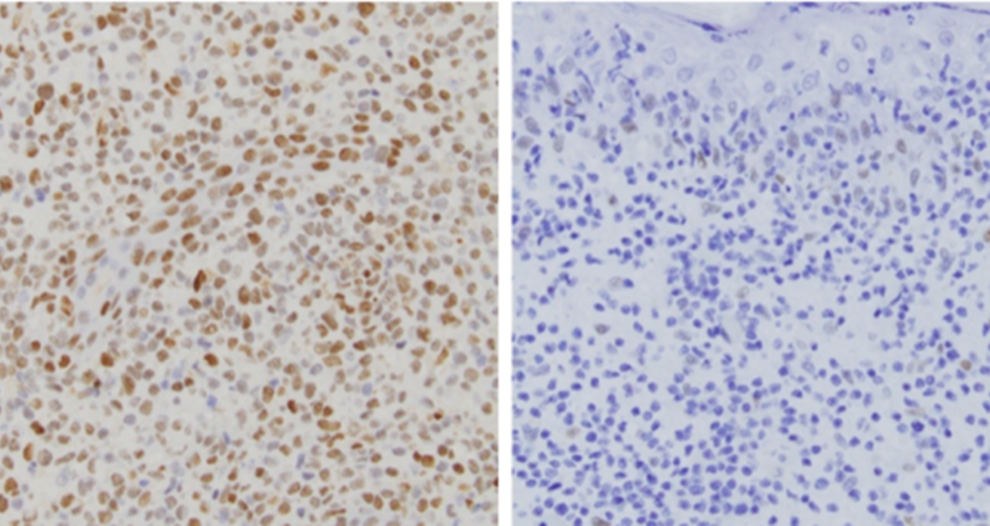
October 2021 Tumor skin biopsy from two different patients. One patient who has a JAK2 gene fusion is positive for pSTAT3 staining, a marker of activated JAK-STAT signaling (left). Another patient who lacks the JAK2 gene fusion is negative for pSTAT3 (right). The patient with the JAK2 fusion is predicted to have good response to JAK2 inhibitors.
SKIN CANCER HARBORS TARGETABLE MUTATIONFeaturing: Jaehyuk Choi, MD, PhD
An especially deadly subtype of T-cell lymphoma is distinguished by unique mutations in a specific protein signaling pathway, according to a study published in the journal Blood. Correcting the downstream effect of these mutations with a pharmacological inhibitor could prove a worthwhile treatment and further showcase the benefits of precision medicine, according to Jaehyuk Choi, MD, PhD, the Jack W. Graffin Professor and senior author of the study. “All patients are different, but we tend to treat them all the same,” said Choi, who is also an associate professor of Dermatology and of Biochemistry and Molecular Genetics. “Our goal is precision medicine. This means we will develop simple molecular tests to identify the best way to treat individual patients.” Cytotoxic cutaneous T-cell lymphoma (CTCL) is a rare but highly aggressive skin cancer. Previous studies have found mutations in the pathway known as JAK-STAT in other blood cancers, but that pathways’ association with CTCL was unknown. In the current study, Choi and his collaborators performed genetic sequencing on archived biopsy samples of CD8+ aggressive CTCL, searching for mutations in the JAK-STAT. The investigators discovered several gene fusion events: whole chromosomes interacting with other chromosomes, all involving JAK2, a kinase in the pathway. “These events are rare to begin with, so the fact that they all associate with JAK2 is astonishing,” said Katie Lee, a research fellow in the Choi laboratory, a medical student at the University of Illinois-Chicago and lead author of the study. Further, these JAK2 mutations were nearly perfectly predictive of the aggressive CD8+ subtype, suggesting that the presence of those mutations could serve as a useful biomarker. “Pathologists can see subtle differences between cancer subtypes under the microscope, and now we can link these differences directly to molecular aberration,” Choi said. JAK2 mutations improve cancer proliferation but these downstream effects could be countered with a JAK2 inhibitor, and a clinical trial utilizing this strategy is already in progress. “The molecular features of the mutation are as such that we have a very good reason to predict complete responses to this therapy,” Choi said. “We think this agent can offer a chance for a cure.” Choi is a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University. This study was supported by National Institute of Arthritis Musculoskeletal and Skin Diseases grant P30-AR066524; National Cancer Institute grants R35-CA231958 and P01-CA248384; Leukemia & Lymphoma Society grant SCOR 7026-21 and Doris Duke Charitable Fund grant 2020132. This article was originally published in the Northwestern University Feinberg School of Medicine News Center on October 7, 2021. |
Jaehyuk Choi, MD, PhD, the Jack W. Graffin Professor and an associate professor of Dermatology and of Biochemistry and Molecular Genetics, was senior author of the study published in Blood.
Refer a PatientNorthwestern Medicine welcomes the opportunity to partner with you in caring for your patients.
|
You May Also Like
|
September 2021 |
September 2021 |