|
March 2020 DRUG COMBINATION MAY REDUCE RISK OF LEUKEMIA RELAPSEFeaturing: Weiqi Huang, MD, PhD and Elizabeth Eklund, MD
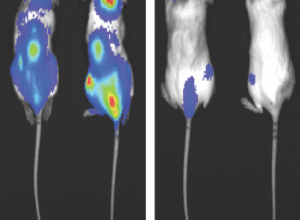
Risk of relapse in chronic myeloid leukemia (CML) may be reduced through a specific combination of drugs — one that inhibits an oncogene and another that inhibits a cell-death protein prevalent in CML cells called Survivin, according to a Northwestern Medicine study published in the journal Leukemia. Weiqi Huang, MD, PhD, research associate professor of Medicine in the Division of Hematology and Oncology, was the first author of the study. Chronic myeloid leukemia (CML) is a rare and slowly progressing blood-cell cancer that begins in bone marrow. Unlike acute myeloid leukemia which is fast-growing, CML slowly progresses and patients typically don’t show symptoms until many years after the disease first develops, prompting patients to experience abrupt sickness and have a higher-than-normal white blood cell count. Main treatment options for CML are targeted therapy drugs known as tyrosine kinase inhibitors, which target an oncogene called BCR-ABL, found in CML cells. Patients are required to take these drugs for the rest of their life. For the few CML patients who progress to acute leukemia, referred to as “blast crisis”, few reliable treatments are available such as chemotherapy, surgery, radiation and stem cell transplants. Previous studies have shown that about half of patients who take tyrosine kinase inhibitors obtain a long standing therapy-free remission and can discontinue all treatment. If they ever stop taking the drug, however, patients can also relapse within the first year and a half after initial diagnosis. The underlying problem, according to Elizabeth Eklund, MD, the Johanna Dobe Professor of Hematology and Oncology and senior author of the study, is that there’s currently no concrete way to predict which CML patients will relapse and which ones won’t. “What we wanted to do for this study was determine characteristics of leukemia cells that predicted relapse and also use it as a preclinical therapeutic testing model,” said Eklund, who is also a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University. For previous research regarding CML cells, the investigators utilized CML mouse models and determined that an increased expression of Survivin — a protein that prevents cell death — was a characteristic of CML cells. “We thought if we could inhibit Survivin, then we might be able to kill some of these remaining CML cells during standard treatment and maybe fewer of the mice would relapse,” Eklund said. For the current study, the investigators treated CML mouse models with either a standard CML targeted therapy drug, a Survivin inhibitor drug, or both. The process was then repeated after 20 weeks. In the mice treated with tyrosine kinase inhibitors, the investigators found abnormalities in the bone marrow cells of the CML mouse models’ bone marrow — specifically in the transcriptome, or the total of all messenger RNA molecules expressed from the genes in a single cell. An increased amount of activity in inflammatory pathways within the cell prevented CML cells from dying and ultimately contributed to relapse. In the mouse models who received both drugs, however, the Survivin inhibitor turned off the inflammatory pathways and ultimately reduced the risk of CML relapse, according to Eklund. Noting the connection between the inflammatory pathways and CML relapse, Eklund said the authors are currently pursuing a study in conjunction with the U.S. Department of Veterans Affairs to identify possible changes in gene expression when CML patients experience a relapse. “What we’re interested in pursuing now is how much of a risk is an episode of infection to somebody who has had leukemia and does it actually preferentially stimulate those leukemia cells in the bone marrow compared to the normal cells, causing them to expand and creating leukemia problems,” Eklund said. This work was supported by the National Institutes of Health R01 grants CA174205; DK098812; DK121354; and U.S. Department of Veterans Affairs grants BX002067 and CX001864. This article was originally published in the Feinberg School of Medicine News Center on March 26, 2020. |
Weiqi Huang, MD, PhD, research associate professor of Medicine in the Division of Hematology and Oncology, was the first author of the study published in the journal Leukemia.
Elizabeth Eklund, MD, the Johanna Dobe Professor of Hematology and Oncology, was senior author of the study.
Refer a PatientNorthwestern Medicine welcomes the opportunity to partner with you in caring for your patients.
|
You May Also Like
|
February 2021 |
September 2020 |